Tofacitinib citrate ke moriana oa ngaka (lebitso la khoebo Xeljanz) qalong e entsoeng ke Pfizer bakeng sa sehlopha sa oral Janus kinase (JAK) inhibitors. E ka thibela JAK kinase ka mokhoa o ikhethileng, e thibela litsela tsa JAK / STAT, 'me ka hona e thibela phetisetso ea lisele tsa sele le polelo e amanang le liphatsa tsa lefutso le ts'ebetso, e sebelisetsoang ho phekola ramatiki ea lefu la masapo, ramatiki ea psoriatic, lefu la ulcerative le mafu a mang a ho itšireletsa mafung.
Setlhare se kenyelletsa mefuta e meraro ea litekanyetso: matlapa, matlapa a lokolotsoeng ka nako e telele le tharollo ea molomo. Matlapa a eona a ile a amoheloa ka lekhetlo la pele ke FDA ka 2012, 'me foromo ea litekanyetso tsa tokollo e tsitsitseng e ile ea amoheloa ke FDA ka Hlakola 2016. Ke eona ea pele ea ho phekola manonyeletso a lefu la masapo. Yan ke JAK inhibitor e nkiloeng ka molomo hang ka letsatsi. Ka Tšitoe 2019, sesupo se secha sa lithethefatsi tse lokolotsoeng ka nako e telele se ile sa amoheloa hape bakeng sa lefu la ulcerative colitis (UC) le itekanetseng ho isa ho le matla. Ho phaella moo, liteko tsa hona joale tsa 3 tsa kliniki bakeng sa plaque psoriasis li phethiloe, 'me liteko tse ling tse tšeletseng tsa 3 li ntse li tsoela pele, tse amanang le ramatiki e sebetsang ea psoriatic, ramatiki ea bana ba idiopathic, joalo-joalo. Mofuta oa matšoao. Melemo ea matlapa a sa khaotseng a sebetsa nako e telele 'me a hloka ho nooa hanngoe ka letsatsi a thusa ho laola le ho laola mafu a bakuli.
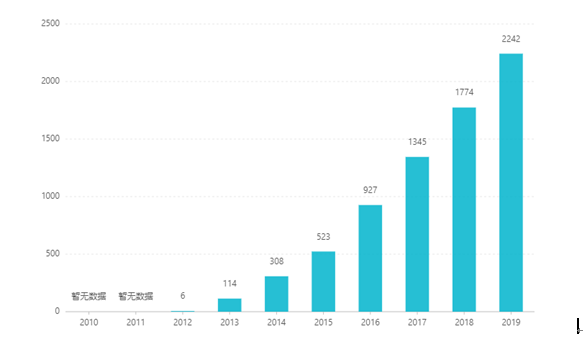
Ho tloha ha e thathamisoa, thekiso ea eona e eketsehile selemo le selemo, e fihla ho US $ 2.242 bilione ka 2019. China, foromo ea litekanyetso tsa letlapa e ile ea amoheloa hore e bapatsoe ka March 2017, 'me e kene lethathamong la inshorense ea bongaka ea B ka lipuisano ka 2019. theko ke RMB 26.79. Leha ho le joalo, ka lebaka la lithibelo tse phahameng tsa tekheniki tsa litokisetso tsa tokollo e tsitsitseng, foromo ena ea litekanyetso ha e so ka e rekisoa Chaena.
JAK kinase e phetha karolo ea bohlokoa ho ruruha, 'me li-inhibitors tsa eona li bontšitsoe ho phekola maloetse a itseng a ho ruruha le a autoimmune. Ho fihlela joale, li-inhibitors tse 7 tsa JAK li amohetsoe lefatšeng ka bophara, ho kenyeletsoa Delgocitinib ea Leo Pharma, Fedratinib ea Celgene, upatinib ea AbbVie, Pefitinib ea Astellas, Baritinib ea Eli Lilly Le Rocotinib ea Novartis. Leha ho le joalo, ke tofacitinib, baritinib le rocotinib feela tse amoheloang Chaena har'a lithethefatsi tse boletsoeng ka holimo. Re lebeletse ka thabo hore "Tofatib Citrate Sustained Release Tablets" ea Qilu e amohetsoe kapele kamoo ho ka khonehang le ho tsoela bakuli ba bangata molemo.
Chaena, lipatlisiso tsa pele tsa tofacitib citrate li amohetsoe ke NMPA ka Hlakubele 2017 bakeng sa kalafo ea bakuli ba baholo ba RA ba nang le ts'ebetso e sa lekaneng kapa ho se mamelle methotrexate, tlasa lebitso la khoebo la Shangjie. Ho latela datha e tsoang ho Meinenet, thekiso ea matlapa a tofacitib citrate litsing tsa bongaka tsa sechaba tsa China ka 2018 e ne e le li-yuan tse limilione tse 8.34, e neng e le tlase haholo ho feta thekiso ea eona ea lefats'e. Karolo e kholo ea lebaka ke theko. Ho tlalehoa hore theko ea pele ea thekiso ea Shangjie e ne e le li-yuan tse 2085 (matlapa a 5mg*28), 'me litšenyehelo tsa khoeli le khoeli e ne e le li-yuan tse 4170, e seng moroalo o monyenyane bakeng sa malapa a tloaelehileng.
Leha ho le joalo, ho bohlokoa ho keteka hore tofacitib e kenyelelitsoe ho "Inshorense ea Naha ea Motheo ea Kalafo, Inshorense ea Kotsi ea Mosebetsi le Lethathamo la Lithethefatsi tsa Maternity Insurance" ea 2019 ke National Medical Insurance Administration ka mor'a lipuisano ka November 2019. Ho tlalehoa hore tefiso ea khoeli le khoeli e tla fokotsoa. ho ea tlase ho li-yuan tse 2,000 ka mor'a hore ho buisanoe ka ho fokotsa theko, e leng ho tla ntlafatsa haholo ho fumaneha ha moriana.
Ka Phato 2018, Boto ea Tlhahlobo ea Patent ea Ofisi ea Naha ea Bohlale e ile ea etsa qeto ea tlhahlobo No. 36902 kopo ea ho se sebetse, 'me ea phatlalatsa hore patent ea mantlha ea Pfizertofatib, e leng kompone ea patent, ha e nepahale, ka lebaka la ho se hlahisoe ho lekaneng ha lintlha. Leha ho le joalo, patent ea Pfizertofatiib crystal form (ZL02823587.8, CN1325498C, letsatsi la kopo 2002.11.25) e tla fela ka 2022.
Database ea Insight e bonts'a hore, ntle le liphuputso tsa mantlha, litlhare tse hlano tsa generic tsa Chia Tai Tianqing, Qilu, Kelun, Yangtze River le Nanjing Chia Tai Tianqing li amohetsoe bakeng sa ho bapatsa letlapeng la lapeng la tofacitinib. Leha ho le joalo, bakeng sa mofuta oa letlapa le lokolotsoeng ka nako e telele, ke phuputso ea pele feela ea Pfizer e kentseng kopo ea ho bapatsa ka May 26. Qilu ke k'hamphani ea pele ea lehae ea ho kenya kopo ea papatso bakeng sa tlhahiso ena. Ho feta moo, CSPC Ouyi e boemong ba teko ea BE.
Changzhou Pharmaceutical Factory (CPF) ke moetsi ea ka sehloohong oa meriana ea li-API, tse phethiloeng ho etsoa China, tse Changzhou, profinseng ea Jiangsu. CPF e thehiloe ka 1949. Re inehetse ho Tofacitinib Citrate ho tloha 2013, 'me re rometse DMF e se e ntse e le teng. Re ngolisitse linaheng tse ngata, 'me re ka u tšehetsa ka litokomane tse molemo ka ho fetisisa tse tšehetsang Tofacitinib Citrate.
Nako ea poso: Jul-23-2021